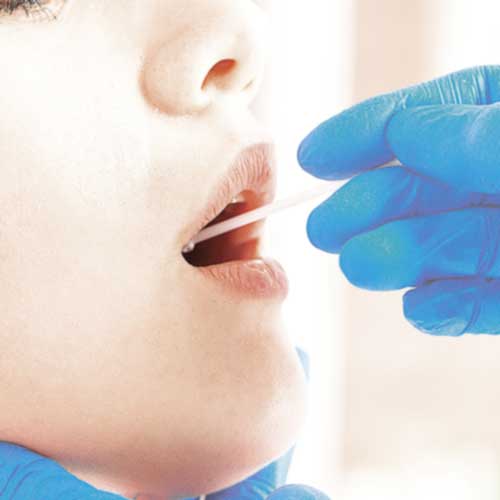
EDELVITAL™ is pleased to announce its new 3in1 Rapid COVID-19 Antigen test for the detection of SARS-CoV-2 in adults and children. The EDELVITAL™ 3in1 Rapid Test is registered for both professional and layman use in Austria, and is specially designed for ease of use. It can be used as “lollipop” test for children by placing the swab on the tongue after coughing and allowing it to absorb saliva (saliva test). The swab can also be placed in the first 2.5cm of the anterior part of the nasal passage (nasal test), or the throat (oropharyngeal test). If necessary it can also be used anally (anal test) during hospitalisation.
The antigen test also detects antigens of the SARS-CoV-2 Omikron variant (B.1.1.529), the Delta variant (B.1617.2), the Beta variant 501.V2 (N501Y.V2 or B.1.351) and the Alpha variant of the corona virus VOC-202012/01 (also 20I / 501Y .V1 or B.1.1.7 or B117).
Ideal for children &
the elderly.
The EDELVITAL™ 3 in 1 Rapid COVID-19 Antigen test is one of the mostly highly accurate on the market. As approved by the Paul Ehrlich Institute in Germany, and registered with the German Medical regulator BfArM, with a specificity of 100%. The less invasive test for children and the elderly can be used by placing the swab on the tongue after coughing and allowing it to absorb oral saliva, sometimes referred to as the “Lollipop test”.
Antigen Tests

3in1 Rapid COVID-19 Antigen Test Kit (Colloidal Gold)
Kit
1
Tests
20


3in1 Rapid COVID-19 Antigen Test (Colloidal Gold)
Pcs
1
Test
1
EDELVITAL™ 3in1 Rapid COVID-19 Antigen Test kit
components
3 in 1 specimen collection
CE, EU IVD certified &
BfArM & GÖG listed
CE – IVD Certification
EDELVITAL ™ COVID-19 tests are CE certified
according to 98/79 guidelines for medical in vitro diagnostics, appendix III, and
are listed at BfArM Germany & GÖG Austria.
Applicable standards:
ISO 13485: 2016, ISO 14971: 2019, EN ISO 18113-1: 2011, EN ISO 18113-2: 2011,
EN ISO 18113-3: 2011, EN 13641: 2002, ISO 15223-1: 2016,
EN 13612: 2002, ISO 23640: 2015, EN 62366-1: 2015

Rapid COVID-19 Antigen 3 in 1 Test
Unique features
The unique features of this test in comparison to other Antigen tests on the market is five-fold.