Essential testing
With this new Point-of-Care Neutralizing Antibody Fluorescence, Immunochromatographic Assay test, EDELVITAL™, offers for the first time, the unique opportunity, for patients that have either previously contracted the SARS CoV-2 virus, or have been vaccinated, to finally have a quantitative status of their immunogenicity, in relation to this disease and their subsequent ability to fight the infection.
A passport solution
This vital information, used together with our proprietary software can aid institutions looking for a passport solution, for access and travel control. It can also inform on quarantine periods and provide a far more accurate reflection of community Infectivity status, than inoculation alone.
A passport solution
This vital information, used together with our proprietary software can aid institutions looking for a passport solution, for access and travel control. It can also inform on quarantine periods and provide a far more accurate reflection of community Infectivity status, than inoculation alone.
What is a neutralizing
antibody of SARS-CoV-2?
The SARS-CoV-2 Neutralizing Anitbody is a special type of antibody produced in a particular part of the body with a unique function. Additionally known as an inhibitive or BLOCKING antibody, the quantity of SARS-CoV-2 Neutralizing antibodies in the blood stream is directly linked to the ability to fight an infection of the disease.
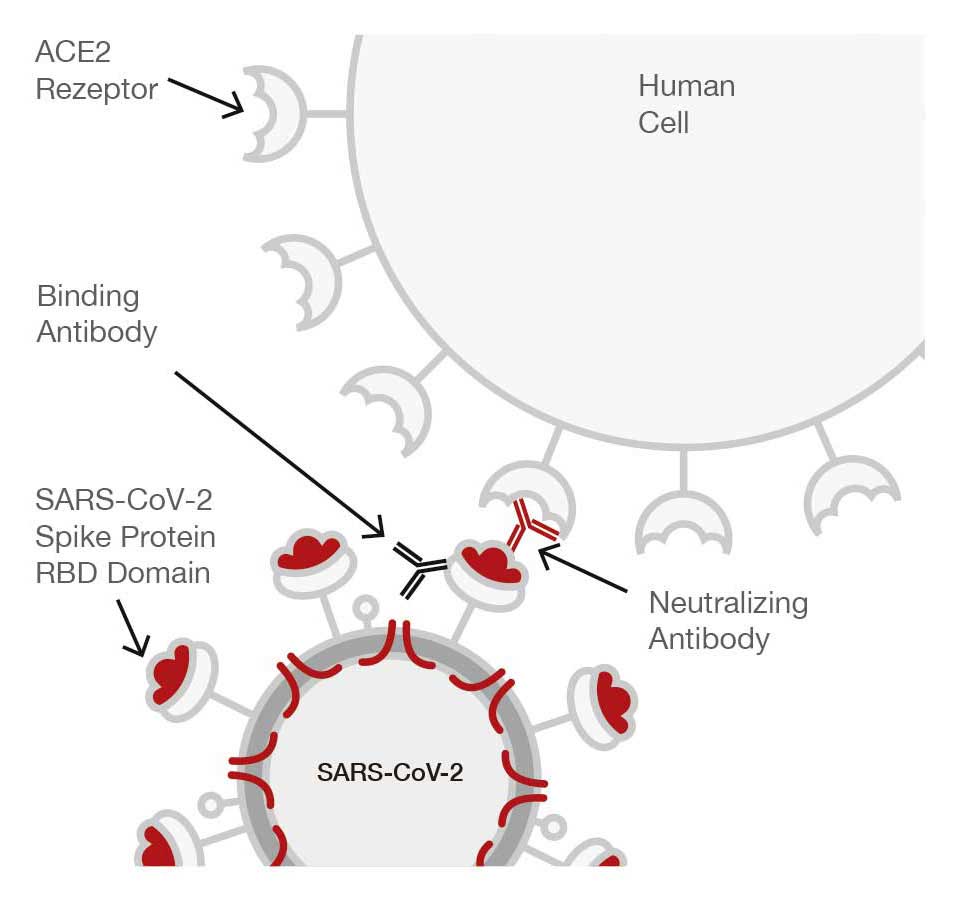
Why and how are people infected by the SARS-CoV-2 virus?
Patients become infected when the RBD (receptor binding domain of the glycoprotein viral spike) interacts with the ACE 2 cell surface receptor of the patient’s lung, directly initiating an infection.

As an advanced point of care solution, the test has undergone clinical trials with a cohort of over 3350 people, in a comparative clinical study, against both ELISA as well as PRNT tests for SARS CoV-2 Neutralizing antibodies (NAb). PRNT (Plaque reduction neutralization test ) is the gold standard test worldwide, for quantifying the titer value of neutralising antibodies for a virus in blood.
Neutralizing antibodies (NAb) of SARS-CoV-2, are those antibodies that can block the interaction of the RBD on the protein spike with the ACE 2 receptor on a host cell (recognised by WHO as a measurement in IU/mL) to prevent the virus reaching the lung. Following the trial a cut-off value of 25 IU/mL was determined to be the titer value, that prevented the infection of the SARS-CoV-2 virus from entering the patients lung, and the Edelvital neutralising antibody of SARS CoV-2 has been calibrated to this PRNT value.
The difference between Neutralizing Antibodies (NAb) and Binding antibodies (BAU)
The difference between Neutralizing Antibodies (NAb) and Binding antibodies (BAU)
It is scientifically recognised that the target of the virus is the patient’s lung. In addition, when the RBD of the viral spike has successfully docked onto the ACE 2 receptor cell of the lung, that infection has been initiated (Yan et al., 2020 Wrapp et al., Tai et al., 2019).
Only Neutralizing antibodies or BLOCKING antibodies, therefore, that operate differently from binding antibodies and cover the small ACE 2 entrance receptors of the lung cells, prevent the virus from entering the lung and are important when making an assessment of infectivity.
The EDELVITAL™ Neutralizing antibody test was designed following a clinical trial of 3350 people utilising the gold standard PRNT test the results for which, the machine is calibrated. The only test of its kind currently being used for further investigations by healthcare professionals. Including a study at the AKH in collaboration with AGIS and the Medical University of Vienna. It is the best point of care test, to assist healthcare professionals in making that assessment.

Binding antibodies attempting to neutralize the RBD from docking at the ACE2 receptor.
RBD can still successfully dock to the ACE2 receptor
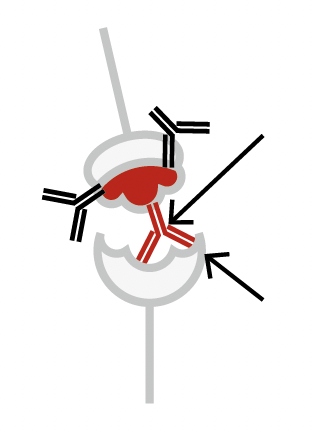
Neutralizing (Blocking) antibody
RBD successfully blocked from docking to the ACE2 receptor
EDELVITAL™ Neutralizing Antibody of SARS-CoV-2 Test kit
components
Application
Clear results in
under 15 seconds
Following preparation, the EDELVITAL ™ Point-of-Care analyzer provides a precise quantitative result for a professional assessment of the immunogenicity of the individual. The EDELVITAL ™ Point-of-Care analyser can assess up to 240 tests per hour.
Calibrated in accordance with the WHO International Standards for SARS-CoV-2 antibody ( human) NiBSC Code: 20/136 in IU/mL.
Instruction video
Introducing the new Edelvital Neutralizing Antibody of SARS-CoV-2 Test (NAb)
(Fluorescence Immunochromatographic Assay)
Download